
Global Congenital Hyperinsulinism Treatment Market Set to Surpass USD 201.4 Million by 2035 | FMI
Congenital Hyperinsulinism Treatment Market
Rising Prevalence of Genetic Disorders, Technological Advances, and Growing Government Support Power the Market Forward
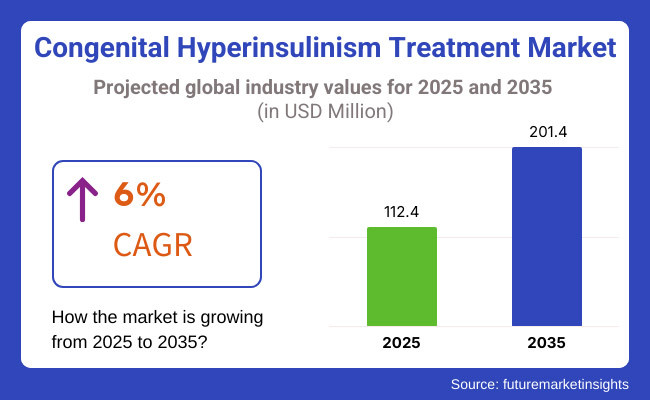
NEWARK, DE, UNITED STATES, April 29, 2025 /EINPresswire.com/ -- The global congenital hyperinsulinism treatment market is poised for robust expansion, with projections indicating growth from USD 112.4 million in 2025 to a remarkable USD 201.4 million by 2035, marking a compound annual growth rate (CAGR) of 6%. This substantial growth trajectory is fueled by a confluence of medical innovation, increasing disease awareness, and global efforts to prioritize rare disease treatment.
Congenital hyperinsulinism, a rare yet serious genetic disorder characterized by the excessive production of insulin in newborns, is drawing increasing attention from healthcare systems, researchers, and biopharmaceutical companies. As global healthcare landscapes evolve, so too does the market potential for targeted treatment solutions that address this complex disorder.
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐊𝐞𝐲 𝐓𝐫𝐞𝐧𝐝𝐬 𝐢𝐧 𝐭𝐡𝐞 𝐌𝐚𝐫𝐤𝐞𝐭: 𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐘𝐨𝐮𝐫 𝐒𝐚𝐦𝐩𝐥𝐞 𝐑𝐞𝐩𝐨𝐫𝐭! https://www.futuremarketinsights.com/report-sample#5245502d47422d3136333631
𝐃𝐫𝐢𝐯𝐞𝐫𝐬 𝐨𝐟 𝐌𝐚𝐫𝐤𝐞𝐭 𝐆𝐫𝐨𝐰𝐭𝐡
Several macro and microeconomic factors are propelling the growth of the CHI treatment market:
• 𝐑𝐢𝐬𝐢𝐧𝐠 𝐏𝐫𝐞𝐯𝐚𝐥𝐞𝐧𝐜𝐞 𝐨𝐟 𝐆𝐞𝐧𝐞𝐭𝐢𝐜 𝐃𝐢𝐬𝐨𝐫𝐝𝐞𝐫𝐬: As genetic testing becomes more accessible, more cases of CHI are being identified globally.
• 𝐀𝐝𝐯𝐚𝐧𝐜𝐞𝐬 𝐢𝐧 𝐆𝐞𝐧𝐞 𝐓𝐡𝐞𝐫𝐚𝐩𝐲: Innovation in gene editing and therapy is revolutionizing how rare diseases like CHI are treated, paving the way for targeted, long-term solutions.
• 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐚𝐧𝐝 𝐃𝐞𝐯𝐞𝐥𝐨𝐩𝐦𝐞𝐧𝐭 𝐈𝐧𝐯𝐞𝐬𝐭𝐦𝐞𝐧𝐭: Increasing global investments in drug development and rare disease research are accelerating the development of effective CHI treatment options.
• 𝐈𝐦𝐩𝐫𝐨𝐯𝐞𝐝 𝐍𝐞𝐨𝐧𝐚𝐭𝐚𝐥 𝐒𝐜𝐫𝐞𝐞𝐧𝐢𝐧𝐠 𝐚𝐧𝐝 𝐄𝐚𝐫𝐥𝐲 𝐃𝐢𝐚𝐠𝐧𝐨𝐬𝐢𝐬: Government-supported screening programs and advocacy campaigns are enabling early identification and intervention, significantly improving treatment outcomes.
• 𝐇𝐞𝐚𝐥𝐭𝐡𝐜𝐚𝐫𝐞 𝐈𝐧𝐟𝐫𝐚𝐬𝐭𝐫𝐮𝐜𝐭𝐮𝐫𝐞 𝐢𝐧 𝐄𝐦𝐞𝐫𝐠𝐢𝐧𝐠 𝐌𝐚𝐫𝐤𝐞𝐭𝐬: Growing healthcare expenditures, particularly in Asia-Pacific and Latin America, are enhancing treatment availability and access.
𝐁𝐚𝐫𝐫𝐢𝐞𝐫𝐬 𝐭𝐨 𝐆𝐫𝐨𝐰𝐭𝐡
Despite the promising future, the market still faces several challenges:
• 𝐇𝐢𝐠𝐡 𝐓𝐫𝐞𝐚𝐭𝐦𝐞𝐧𝐭 𝐂𝐨𝐬𝐭𝐬: Innovative therapies and specialized treatments often come with significant costs, limiting accessibility.
• 𝐒𝐡𝐨𝐫𝐭𝐚𝐠𝐞 𝐨𝐟 𝐓𝐫𝐚𝐢𝐧𝐞𝐝 𝐒𝐩𝐞𝐜𝐢𝐚𝐥𝐢𝐬𝐭𝐬: Effective CHI management requires expert endocrinologists and pediatric specialists, which are lacking in many regions.
• 𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐨𝐫𝐲 𝐂𝐨𝐦𝐩𝐥𝐞𝐱𝐢𝐭𝐢𝐞𝐬: Navigating orphan drug approvals and variable regulations across countries can delay product launches and patient access.
Nonetheless, ongoing clinical trials, strategic collaborations, and efforts to decentralize care are gradually addressing these bottlenecks.
𝐒𝐨𝐚𝐫𝐢𝐧𝐠 𝐃𝐞𝐦𝐚𝐧𝐝 𝐟𝐨𝐫 𝐌𝐚𝐫𝐤𝐞𝐭 𝐈𝐧𝐟𝐨𝐫𝐦𝐚𝐭𝐢𝐨𝐧: 𝐔𝐧𝐜𝐨𝐯𝐞𝐫 𝐃𝐞𝐭𝐚𝐢𝐥𝐞𝐝 𝐓𝐫𝐞𝐧𝐝𝐬 𝐚𝐧𝐝 𝐈𝐧𝐬𝐢𝐠𝐡𝐭𝐬 𝐢𝐧 𝐎𝐮𝐫 𝐑𝐞𝐩𝐨𝐫𝐭! https://www.futuremarketinsights.com/reports/congenital-hyperinsulinism-treatment-market
𝐂𝐨𝐮𝐧𝐭𝐫𝐲-𝐖𝐢𝐬𝐞 𝐌𝐚𝐫𝐤𝐞𝐭 𝐎𝐮𝐭𝐥𝐨𝐨𝐤: 𝐊𝐞𝐲 𝐓𝐚𝐤𝐞𝐚𝐰𝐚𝐲𝐬
𝐔𝐧𝐢𝐭𝐞𝐝 𝐒𝐭𝐚𝐭𝐞𝐬 (𝐂𝐀𝐆𝐑: 𝟓.𝟖%)
Leading the global market, the U.S. benefits from a robust healthcare infrastructure, expansive research funding, and active organizations like the Congenital Hyperinsulinism International (CHI) Foundation that drive awareness and early diagnosis. Government backing for orphan drug development continues to open new avenues for treatment.
𝐔𝐧𝐢𝐭𝐞𝐝 𝐊𝐢𝐧𝐠𝐝𝐨𝐦 (𝐂𝐀𝐆𝐑: 𝟓.𝟒%)
Support from the National Health Service (NHS), precision medicine initiatives, and newborn screening programs position the UK for steady market growth. Centers like Great Ormond Street Hospital provide specialized care, making the UK a significant contributor to CHI innovation in Europe.
𝐄𝐮𝐫𝐨𝐩𝐞𝐚𝐧 𝐔𝐧𝐢𝐨𝐧 (𝐂𝐀𝐆𝐑: 𝟓.𝟔%)
Germany, France, and Spain are key players within the EU, benefiting from the European Medicines Agency (EMA)’s orphan drug incentives and progressive healthcare reforms. Cross-border collaborations between research institutes and biotech firms are driving diagnostics and therapy advancements.
𝐉𝐚𝐩𝐚𝐧 (𝐂𝐀𝐆𝐑: 𝟓.𝟓%)
Japan is rapidly emerging as a hub for personalized medicine and gene therapies. With government incentives for rare disease treatment and adoption of next-generation sequencing (NGS), Japan is poised to lead CHI innovation in Asia.
𝐒𝐨𝐮𝐭𝐡 𝐊𝐨𝐫𝐞𝐚 (𝐂𝐀𝐆𝐑: 𝟓.𝟕%)
South Korea’s increasing healthcare expenditure, neonatal care upgrades, and rare disease research investments are accelerating CHI treatment capabilities. Academic and biotech collaborations are helping translate R&D into clinical solutions at a faster pace.
𝐑𝐞𝐜𝐞𝐧𝐭 𝐈𝐧𝐝𝐮𝐬𝐭𝐫𝐲 𝐃𝐞𝐯𝐞𝐥𝐨𝐩𝐦𝐞𝐧𝐭𝐬 & 𝐌𝐚𝐫𝐤𝐞𝐭 𝐓𝐫𝐞𝐧𝐝𝐬
The CHI treatment market is witnessing dynamic activity through:
• Development of Pancreatic Targeting Drugs: Offering better outcomes with reduced systemic effects.
• Shift Toward Minimally Invasive Surgery: Surgical options for CHI are becoming more refined, with less risk and quicker recovery.
• Adoption of Precision Medicine: Tailoring treatment protocols to individual genetic profiles is becoming a standard of care.
• Emergence of Patient Advocacy Groups: Increasing public and governmental attention to CHI through education and lobbying.
𝐒𝐭𝐚𝐲 𝐀𝐡𝐞𝐚𝐝 𝐨𝐟 𝐇𝐞𝐚𝐥𝐭𝐡𝐜𝐚𝐫𝐞 𝐌𝐚𝐫𝐤𝐞𝐭 𝐃𝐢𝐬𝐫𝐮𝐩𝐭𝐢𝐨𝐧𝐬! https://www.futuremarketinsights.com/industry-analysis/therapy-area
𝐂𝐨𝐦𝐩𝐞𝐭𝐢𝐭𝐢𝐯𝐞 𝐋𝐚𝐧𝐝𝐬𝐜𝐚𝐩𝐞
Numerous biopharmaceutical companies are intensifying their focus on congenital hyperinsulinism, driving a competitive and innovation-centric market. Key players include:
• Eli Lilly
• Novo Nordisk
• Novartis AG
• Rezolute, Inc.
• Eiger Biopharmaceuticals
• Hanmi Pharmaceutical Co., Ltd.
• Crinetics Pharmaceuticals, Inc.
• AmideBio LLC
• Xeris Pharmaceuticals, Inc.
• Zealand Pharma A/S
These companies are actively pursuing drug development pipelines, partnerships with research institutes, and regulatory approvals for novel treatments.
𝐌𝐚𝐫𝐤𝐞𝐭 𝐒𝐞𝐠𝐦𝐞𝐧𝐭𝐚𝐭𝐢𝐨𝐧
The CHI treatment market can be segmented as follows:
𝗕𝘆 𝗧𝗿𝗲𝗮𝘁𝗺𝗲𝗻𝘁 𝗠𝗲𝘁𝗵𝗼𝗱:
• Drug Therapy
• Surgery
𝗕𝘆 𝗔𝗽𝗽𝗹𝗶𝗰𝗮𝘁𝗶𝗼𝗻:
• Hospitals
• Clinics
• Others
𝗕𝘆 𝗥𝗲𝗴𝗶𝗼𝗻:
• North America
• Latin America
• Western Europe
• Eastern Europe
• East Asia
• South Asia Pacific
• Middle East and Africa
𝐋𝐨𝐨𝐤𝐢𝐧𝐠 𝐀𝐡𝐞𝐚𝐝
The congenital hyperinsulinism treatment market is at the cusp of a transformative decade. Fueled by breakthrough research in genomics, increasing public health investments, and stronger advocacy for rare diseases, the sector is expected to deliver both economic value and enhanced patient outcomes. While challenges remain in cost and accessibility, innovation, collaboration, and regulatory support are aligning to ensure a more hopeful future for children and families affected by this rare condition.
Ankush Nikam
Future Market Insights, Inc.
+91 90966 84197
email us here
Visit us on social media:
LinkedIn
Facebook
YouTube
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release